Now, onto the science! As we discussed last time, Prussian Blue is considered the first of all synthetic pigments; as such, there is a lot known about the science behind it! In this article, we’re going to dive a bit deeper into that facet, covering:….
- How Prussian Blue is made
- Why it’s blue
- Why the pigment isn’t lightfast… but companies rate it as if it is
- Why it isn’t toxic in it’s base state.
Don’t worry; I’ve spent weeks reading and re-reading this and getting non-chemist’s opinions on the article to make sure your eyes won’t bleed while reading it. However, I am /always/ happy to discuss chemistry, pigments, and art, and so if you have any questions, please comment below!
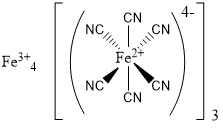
To start with, this is what a graphical representation of Prussian Blue looks like. You don’t need to know this, however sometimes it is helpful to have an idea of what makes up our pigments. You can see that there are 7 iron atoms per molecule, 18 carbons, and 18 nitrogens (these in the form of cyanide anions). As the molecule includes inorganic atoms (the iron, a metal in this case), Prussian Blue is considered an inorganic pigment.

Synthesis
Okay, I’m going to be a bit serious here first and give you all a safety notice. There are videos on youtube of people creating Prussian Blue pigment. Whilst it is technically possible to make this colour at home, I would highly reccomend against this. All chemical reactions should be performed in ventilated fume hoods, and with appropriate protective equipment and safety assessments. The information below is presented to give you an appreciation of how the pigment is made, rather than full on instructions as to how to do it yourself. I welcome discussion in the comments below, provided you respect the dangers of creating pigments without appropriate training and supervision.
Right! Onto the fun stuff!
There are two main ways to make this pigment: the direct way and the indirect way.
The direct approach is simpler, and is done by adding a solution of iron(III) chloride (one iron atom with three chlorines bound to it) to potassium ferrocyanide at room temperature (see formula below).

The indirect approach however goes via the potassium or sodium salt of Prussian Blue (otherwise known as Berlin White, made from iron (II) salts) which is then oxidised using hydrogen peroxide.
Berlin white in this case is the white sodium/potassium hexacyanoferrate salt. As all the iron is in the same oxidation state (see below for more information), the compound is white. The name is also used to describe white lead, when it is used as a pigment.

Solubility
It should be noted that while many refer to the two variant products as ‘soluble’ and ‘insoluble’ Prussian Blues, both are in fact insoluble – it’s a historical term that stuck. Instead, think of it as if the ‘soluble’ form is just more readily able to disperse in aqueous solutions. The primary influence as to whether these compounds are regarded as ‘soluble’ or ‘insoluble’ is when excess iron (III) chloride is added.
To put this into context for fellow paint makers: you know the little ‘strings’ of Prussian Blue that you can get that are a pain to get out? They are called colloids, and occur in the ‘insoluble’ form of Prussian Blue. Sonication (i.e. passing an electric current through to make the particles vibrate and separate) will break these up, however for us, grinding a hella lot does the trick. So depending upon your supplier, and how they make the pigment, you might see these or not! To my knowledge, there isn’t explicit information on whether a pigment is made one way or another, but I will keep an eye on this for you all!
Also of interest… did you know that at one point in history, Prussian Blue wasn’t vegetarian friendly? Prior to the mid 19th century, the alkali hexacyano ferrate was produced en mass via the calcination of waste animal matter (i.e. offal or dried blood). Thankfully, now these materials can be isolated from the waste products of gas purification in industrial scale reactions, taking hydrogen cyanide and reacting it with ferrous chloride (aka iron chloride), and the respective salts.

Green Shade Vs Red Shade
This is dependent on the starting material that was used i.e. a sodium salt, or a potassium salt. The former will give the GS, and the latter the RS. Interestingly, the GS varients give more ‘bronzing’ when you lay the colour down.
Factfile
Name: iron (III) hexacyanoferrate (II)
Chemical formula: Fe4[Fe(CN)6]3
Molecular Weight: 859.2 g/mol
Particle size: ca. 0.5 micrometers
Crystal System: cubic
solubility: 6 mg/ml
Why is Prussian Blue… Blue?
The deep blue colour is caused by the electron transfer from an iron (II) to an iron (III) ion, when visible light (of wavelength 700 nm) is absorbed by the molecule. Colour is, for humans, the wavelengths of light that are reflected by an object, rather than those it actually absorbs. Prussian blue absorbs red light, and gives us that nice blue hue.
Lightfast… Or Not?
As early as 1785, it was noted that Prussian Blue is not as permanent as its predecessors (ultramarine blue). Iron blues lose their colour over time. However if they’re kept isolated in the dark, but exposed to the air, then the pigments will reoxidise and “regain” their potent colour. The first recording of this phenomenon was by Franz Fernback in the 1830s.
Further investigation showed that whilst permanence tests of the time used the pigment at full concentration, this was not representative of how the pigment is actually used; as soon as the pigment is mixed with other components, or diluted, the permanence drops dramatically.
But why does this happen? If you remember from the factfile (above), Prussian blue contains iron in two oxidation states, iron (II), and iron (III). For the purpose of this article, think of oxidation states as the number of electrons the iron has/doesn’t have. In this case, iron (II) = iron 2+, where the two electrons an iron atom would normally possess have been abstracted through the reactions above.
It is also known that the smaller the particle of Prussian Blue is, the more rapidly it will fade. This is attributed to the larger surface area of the particle, i.e. more of it is exposed to the light. It is an electrochromatic pigment, meaning that the colour changes upon excitation, in this case by light. In the presence of light energy, the iron (III) atoms within the Prussian Blue lattice are reduced (gain an electron) to iron (II). When this happens, the metal-to-metal charge transfer between non-equivalent ions that causes the colour cannot occur.
Conversely, when no longer exposed to the light, there is nothing to prevent the reverse of this reaction occuring, and so the colour returns. Note: this is also occurring when exposed to the light, however the process is dominated by the reduction, and so overall the fading occurs. This is why paintings must be stored for extended periods of time to have their colours restored to their former glory.
In Summary
The reaction with light occurs quicker than that with oxygen, hence, the colour fades.
LIGHT makes the colour fade.
OXYGEN brings the colour back.
Toxicity
In terms of whether the pigment is toxic… not very, provided you’re careful. Prussian Blue is used to treat heavy metal poisoning, particularly after the Chernobyl nuclear reactor accident, and studies since have shown that no toxic effects were noted in humans (see Pearce’s article, citation below,
for more information).
It is another case however if you decide to burn your paint! The thermal stability of Prussian Blue decreases rapidly from around 250 degrees Celsius, releasing cyanide into the atmosphere. So folks, don’t go around setting fire to your paints.
Use as a medicine
Prussian Blue is on the WHO’s list of essential medicines, a collection of the safest and most effective medicines needed. It helps alleviate heavy metal poisoning by binding to the metal (thallium or caesium) and preventing their absorption in the intestines.
Next time, I will be going a little into the colour, and how it compares to other blues that we commonly use!
References
- J. Pearce, Food Chem. Toxicol., 1994, 32, 6, 577-582
- Aparicio, C., Machala, L. & Marusak, Z., J Therm Anal Calorim, 110, 661–669 (2012). https://doi.org/10.1007/s10973-011-1890-1
- L. Samain et. al., J. Phys. Chem., 2013, 117, 9693-9712

2 Comments
Ilse Åsbakk
This was such an interesting read! I learned so many things I didn’t know I wanted to learn! The lightfastness issue has been a mystery to me, but this explained it exactly detailed enough for my brain to go “oh, okay, that makes sense!”
khannahart
I’m glad it was understandable and you enjoyed it! ^^